
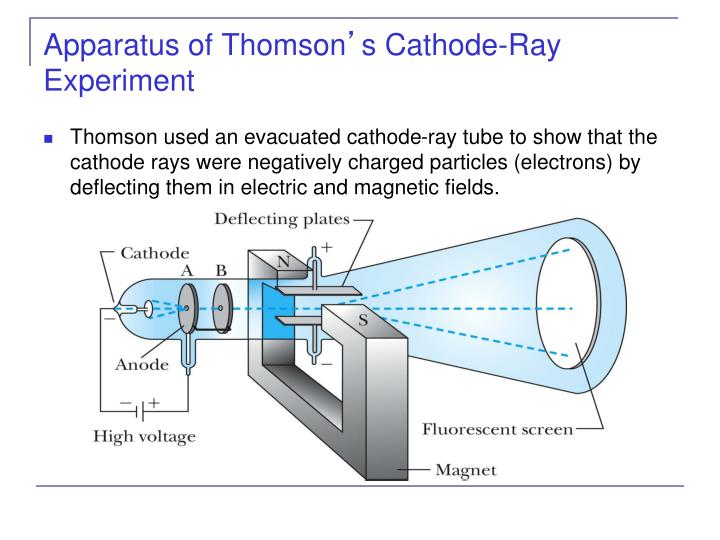
Then Thomson measured how much various strengths of magnetic fields bent the particles.

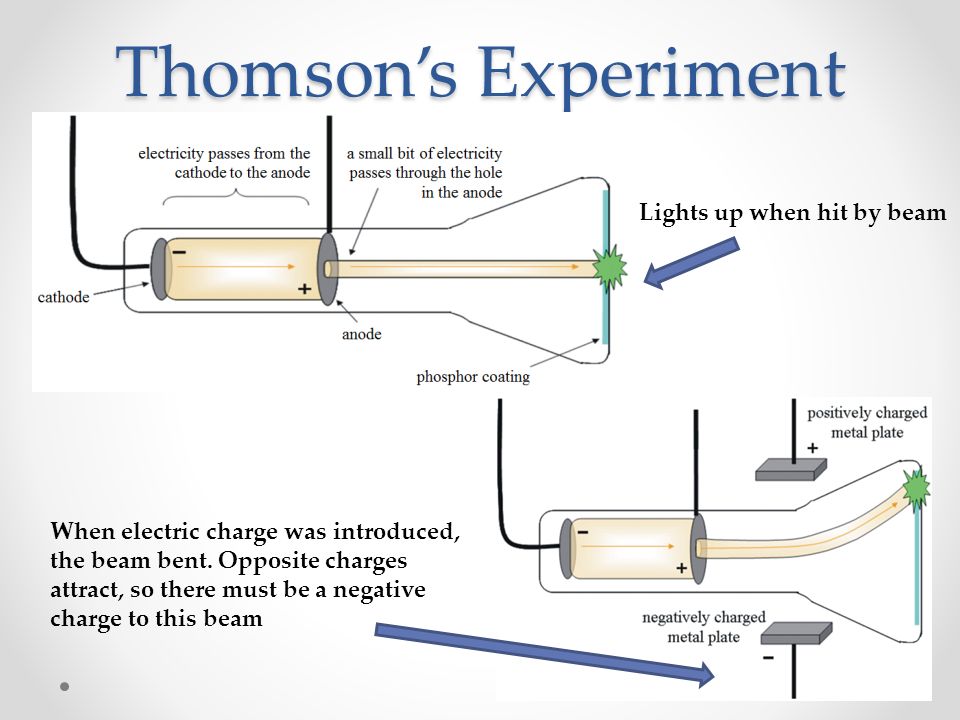
Thomson was able to deflect the cathode ray towards a positively charged plate deduce that the particles in the beam were negatively charged. Thomson also determined the mass to charge ratio of the electron using a cathode ray tube, another significant discovery. Cathode rays are formed when electrons emitted from one electrode and travel to another when a voltage is applied in a vacuum. Thomson determined that charged particles much lighter than atoms, particles that we now call electrons made up cathode rays. Cathode rays played a critical role in unlocking this mystery. Yet until Thomson, no one had determined what these might be. Prior to the discovery of the electron, several scientists had posited that atoms might be made up of smaller pieces. What is a cathode ray tube and why was it important? For instance, the discovery of the electron was vital to the development of chemistry today, and it was the first subatomic particle to be discovered. Although he was awarded the Nobel Prize in physics and not chemistry, Thomson’s contributions to the field of chemistry are numerous. Thomson did most of this work while leading the famed Cavendish Laboratory at the University of Cambridge. In addition to this work, Thomson also performed the first-ever mass spectrometry experiments, discovered the first isotope and made important contributions both to the understanding of positively charged particles and electrical conductivity in gases. Thompson made the switch to physics a few years later and began studying the properties of cathode rays. Thompson was born in December 1856 in Manchester, England and was educated at the University of Manchester and then the University of Cambridge, graduating with a degree in mathematics. They are a development of some experiments of which a preliminary account appeared recently in ‘Nature.JJ Thomson was an English physicist who discovered the electron in 1897. It is hoped that the experiments described in this paper will advance the matter a stage further. Davisson and Kunsman and Davisson and Germer have obtained results on the reflection of slow electrons from the surfaces of crystals, especially nickel, which show good qualitative agreement with the theory but a discrepancy of 30 per cent. Dymond has obtained some remarkable results on the scattering of slow electrons in helium which are of the general nature to be expected in this theory, but our knowledge of the structure of helium, together with the mathematical difficulties of the problem have so far prevented any exact comparision of the theory with experiment. In view, however, of the extremely fundamental nature of the theory it is highly desirable that it should rest on more direct evidence, and, in particular, that it should be shown capable of predicting as well as of merely explaining. The consequences of this theory have been worked out by de Broglie, Schrödinger and others and applied to problems in spectroscopy where they have provided the solution of several outstanding difficulties left by the older theory of orbits.
THOMSON CATHODE RAY EXPERIMENT FOR FREE
The above is for free space in the presence of a field of force V varies, and the consequent bending of the waves by refraction corresponds, on the new theory, to the deviation of the path of the particle by the field of force, on the old. Compare, in ordinary optical theory, the case of substances, such as sodium, for which the refractive index is less than unity. There is nothing impossible in this because the waves are regarded as purely geometrical -“phase waves”-not as carrying energy. Here c is the velocity of light and it will be seen that the wave velocity is greater than c.
In fact if m 0 is the mass for slow speed and v the speed of a freely moving particle, the wave-length is given by λ = h√1- v 2/ c 2 / m 0 v, and the wave velocity V by V = c 2/ v, the group velocity being v, the velocity of the particle. de Broglie has introduced a theory of mechanics according to which a moving particle behaves as a group of waves whose velocity and wave-length are governed by the speed and mass of the particle.


 0 kommentar(er)
0 kommentar(er)
